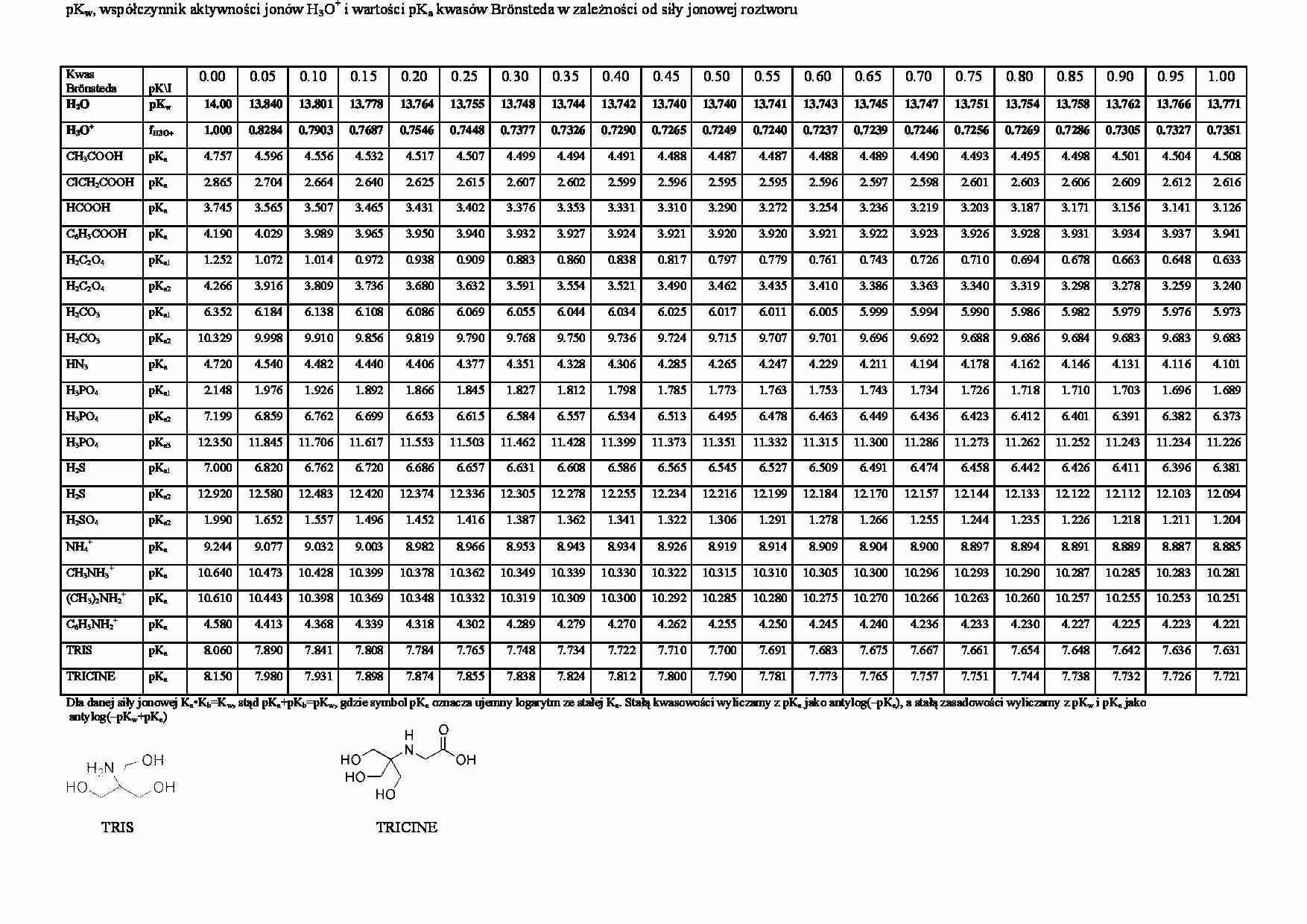
pKw, współczynnik aktywności jonów H3O + i wartości pKa kwasów Brönsteda w zależności od siły jonowej roztworu Kwas Brönsteda pK\I 0.00 0.05 0.10 0.15 0.20 0.25 0.30 0.35 0.40 0.45 0.50 0.55 0.60 0.65 0.70 0.75 0.80 0.85 0.90 0.95 1.00 H2O pKw 14.00 13.840 13.801 13.778 13.764 13.755 13.748 13.744 13.742 13.740 13.740 13.741 13.743 13.745 13.747 13.751 13.754 13.758 13.762 13.766 13.771 H3O + fH3O+ 1.000 0.8284 0.7903 0.7687 0.7546 0.7448 0.7377 0.7326 0.7290 0.7265 0.7249 0.7240 0.7237 0.7239 0.7246 0.7256 0.7269 0.7286 0.7305 0.7327 0.7351 CH3COOH pKa 4.757 4.596 4.556 4.532 4.517 4.507 4.499 4.494 4.491 4.488 4.487 4.487 4.488 4.489 4.490 4.493 4.495 4.498 4.501 4.504 4.508 ClCH2COOH pKa 2.865 2.704 2.664 2.640 2.625 2.615 2.607 2.602 2.599 2.596 2.595 2.595 2.596 2.597 2.598 2.601 2.603 2.606 2.609 2.612 2.616 HCOOH pKa 3.745 3.565 3.507 3.465 3.431 3.402 3.376 3.353 3.331 3.310 3.290 3.272 3.254 3.236 3.219 3.203 3.187 3.171 3.156 3.141 3.126 C6H5COOH pKa 4.190 4.029 3.989 3.965 3.950 3.940 3.932 3.927 3.924 3.921 3.920 3.920 3.921 3.922 3.923 3.926 3.928 3.931 3.934 3.937 3.941 H2C2O4 pKa1 1.252 1.072 1.014 0.972 0.938 0.909 0.883 0.860 0.838 0.817 0.797 0.779 0.761 0.743 0.726 0.710 0.694 0.678 0.663 0.648 0.633 H2C2O4 pKa2 4.266 3.916 3.809 3.736 3.680 3.632 3.591 3.554 3.521 3.490 3.462 3.435 3.410 3.386 3.363 3.340 3.319 3.298 3.278 3.259 3.240 H2CO3 pKa1 6.352 6.184 6.138 6.108 6.086 6.069 6.055 6.044 6.034 6.025 6.017 6.011 6.005 5.999 5.994 5.990 5.986 5.982 5.979 5.976 5.973 H2CO3 pKa2 10.329 9.998 9.910 9.856 9.819 9.790 9.768 9.750 9.736 9.724 9.715 9.707 9.701 9.696 9.692 9.688 9.686 9.684 9.683 9.683 9.683 HN3 pKa 4.720 4.540 4.482 4.440 4.406 4.377 4.351 4.328 4.306 4.285 4.265 4.247 4.229 4.211 4.194 4.178 4.162 4.146 4.131 4.116 4.101 H3PO4 pKa1 2.148 1.976 1.926 1.892 1.866 1.845 1.827 1.812 1.798 1.785 1.773 1.763 1.753 1.743 1.734 1.726 1.718 1.710 1.703 1.696 1.689 H3PO4 pKa2 7.199 6.859 6.762 6.699 6.653 6.615 6.584 6.557 6.534 6.513 6.495 6.478 6.463 6.449 6.436 6.423 6.412 6.401 6.391 6.382 6.373 H3PO4 pKa3 12.350 11.845 11.706 11.617 11.553 11.503 11.462 11.428 11.399 11.373 11.351 11.332 11.315 11.300 11.286 11.273 11.262 11.252 11.243 11.234 11.226 H2S pKa1 7.000 6.820 6.762 6.720
... zobacz całą notatkę



Komentarze użytkowników (0)