To tylko jedna z 8 stron tej notatki. Zaloguj się aby zobaczyć ten dokument.
Zobacz
całą notatkę


Cell Membranes:
Intracellular pH and
Electrochemical Potential
Adam M Gilmore, Australian National University, Canberra City, Australia
Cell metabolism requires transmembrane proton and electrochemical gradients to
synthesize adenosine 5’-triphosphate (ATP), translocate ions, proteins and metabolites and
regulate other vital activities.
Secondary article
Article Contents
. pH Gradients
. Chemiosmotic Hypothesis
. pH Gradient-driven Processes
. Cytochromes, Iron–Sulfur Proteins, Copper Proteins
and Quinones
. Electron Transport and H 1 Translocation
. Redox-driven H 1 Translocation
. Light Energy-driven H 1 Translocation
. ATP Synthesis
. Measurement of DpH and Dw
pH Gradients
Metabolic energy derives from redox-driven oxidation of
nutrients in respiration and/or from light-driven chemical
reactions in photosynthesis. Both processes utilize ‘energytransducing’ membranes, and various associated metalloprotein enzyme complexes and components, to couple
vectorial electron transport to transmembrane proton
translocation. The transmembrane pH gradient
(DpH 5 pHin 2 pHout) provides the cell with energy to
synthesize ATP, which is required for all vital processes.
pH Gradient-driven Processes
ATP synthesizing processes
Chemiosmotic Hypothesis
Eukaryotic respiration: oxidative phosphorylation
Peter Mitchell’s 1978 Nobel Prize winning ‘chemiosmotic
hypothesis’ proposed five points to explain how cell
membranes harness energy for ATP synthesis. First,
energy-transducing membranes form vesicles that separate
aqueous phases inside and outside and resist passive ion or
proton diffusion but not redox-mediated or enzymecatalysed translocation. Second, the proton electrochemical potential gradient consists of two energetically equal
components that can drive ATP synthesis, namely, the
DpH and the electrochemical potential gradient,
Dc 5 cin 2 cout, and is defined as,
Á~Hþ ¼ FÁc À 2:3ðRTÞÁpH
m
conducts protons (F0) and an extrinsic catalytic portion
(F1). ATP hydrolysis forms adenosine diphosphate (ADP),
inorganic phosphate (Pi), and drives an opposing H 1
translocation. The fifth point is that uncouplers dissipate
the pmf by catalysing transmembrane equilibration of H 1
or hydroxyl ions (OH 2 ).
½1
where R is the universal gas constant, T is the absolute
temperature and F is Faraday’s constant. The proton
motive force (pmf 5 D~dF 2 1) is defined in units of volts
m
(V). Third, Figure 1a shows that vectorially alternating
proton (H 1 ) and electron (e 2 ) carriers in an electron
transport chain form the D~d; H 1 deposition occurs on
m
the positive, p-side, in the H 1 –e 2 carrier loop when H 1
carriers carry electrons from the negative, n-side, across the
membrane to the e 2 carriers. Fourth, Figure 1b shows that
the pmf drives the adenosine triphosphatase F-ATPase,
which comprises both a membrane bound portion that
Eukaryotic respiration drives ATP synthesis or ‘oxidative
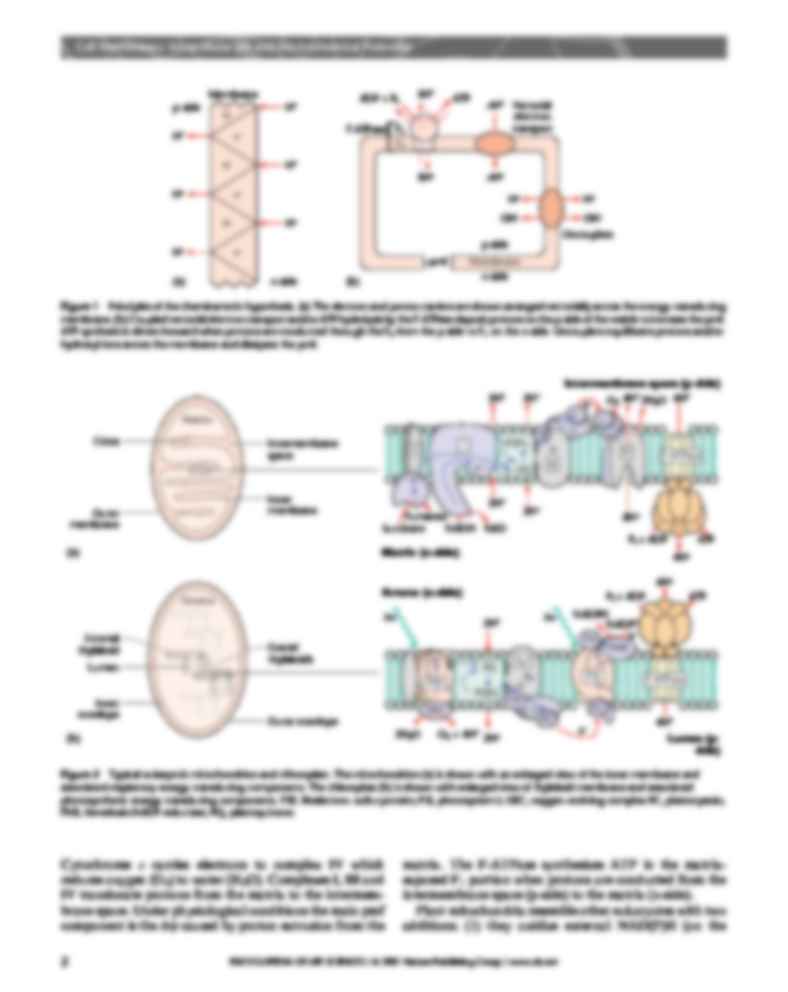
phosphorylation’ in the mitochondria. Figure 2a shows a
typical mitochondrion,
(…)
… the inner membrane and
associated respiratory energy-transducing components. The chloroplast (b) is shown with enlarged view of thylakoid membrane and associated
photosynthetic energy-transducing components. FSP, Rieske iron–sulfur protein; PSI, photosystem I; OEC, oxygen-evolving complex PC, plastocyanin;
FNR, ferredoxin:NADP reductase; PQ, plastoquinone.
Cytochrome c carries electrons to complex IV…
… mitochondria resemble other eukaryotes with two
additions: (1) they oxidize external NAD(P)H (on the
ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net
Cell Membranes: Intracellular pH and Electrochemical Potential
intermembrane space or p-side) using external dehydrogenases to reduce UQ and (2) they have an alternative
oxidase complex in the inner membrane that oxidizes UQ
and…
… respiratory
substrates. Figure 2b shows a typical chloroplast bounded
by an outer and inner envelope surrounding the energytransducing thylakoid membranes. The thylakoids usually
organize as stacks of ‘appressed’ discs or granal thylakoids
connected by the common lumen space to ‘nonappressed’
stromal thylakoids. Thylakoids separate the lumen (p-side)
from the stroma (n-side). The enlarged view in Figure 2b…
… proposed
that they form a ‘dimer of dimers’. Single turnover flashes
of light (one e 2 per flash) drive the OEC through five
oxidation states and water splitting occurs in a concerted
four electron transfer event: 2H2O ! O2 1 4H 1 1 4e 2 .
The four electrons released from the OEC reduce a special
tyrosine residue on the lumen side of PSII that in turn
reduces the oxidized P680 chlorophyll a molecules in PSII…
… succinate (II) in the
matrix and reduce the pool of ubiquinone (UQ) dissolved
in the membrane’s hydrophobic interior. Reduced UQ
picks up protons from the matrix to form UQH2, which
carries electrons and protons to complex III. Complex III
comprises three main proteins (cytochrome b, the ‘Rieske’
iron–sulfur protein (FSP) and cytochrome c1), extracts
electrons from UQH2, translocates protons into the…
... zobacz całą notatkę






Komentarze użytkowników (0)