To tylko jedna z 34 stron tej notatki. Zaloguj się aby zobaczyć ten dokument.
Zobacz
całą notatkę
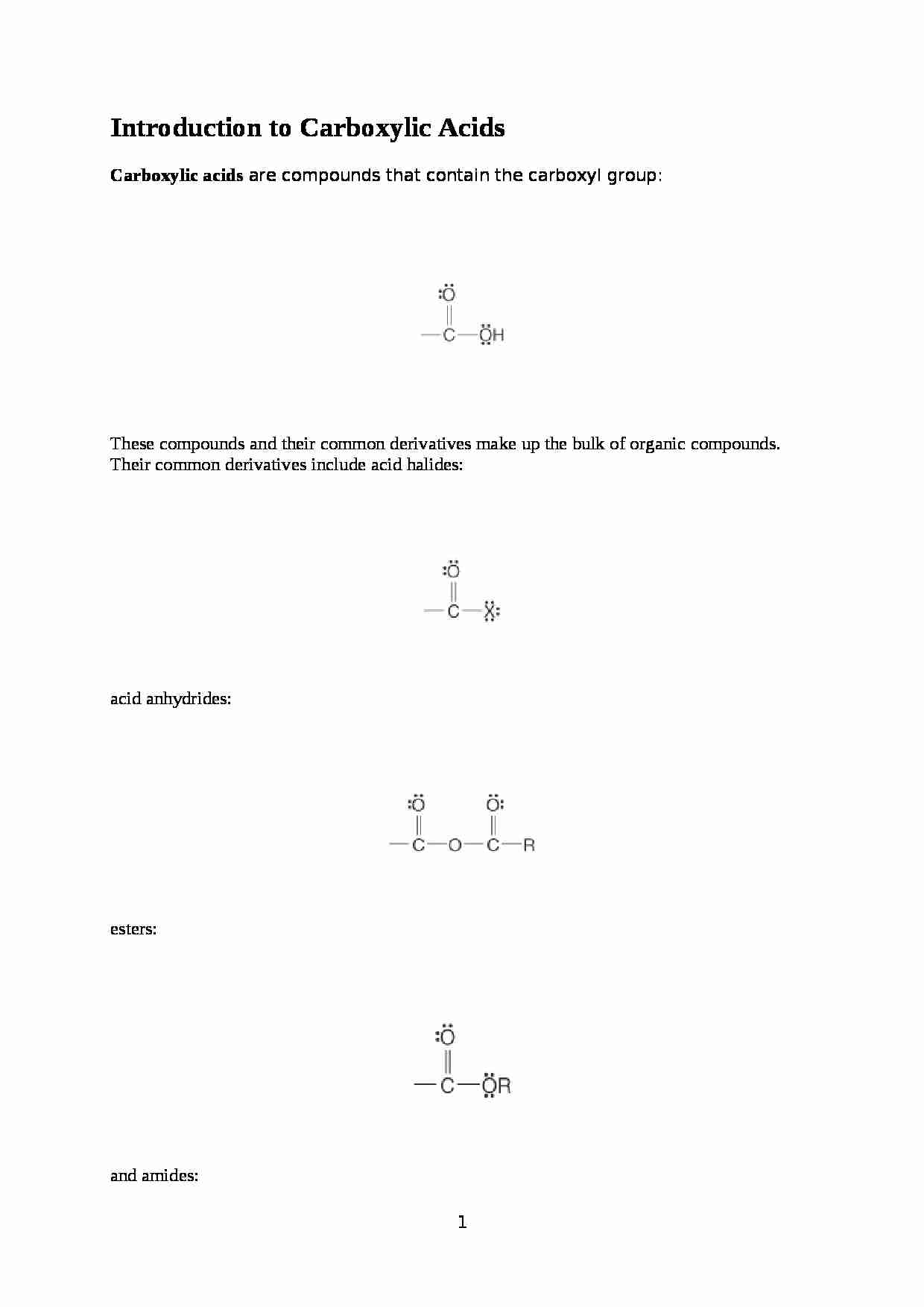


Introduction to Carboxylic Acids
Carboxylic acids are compounds that contain the carboxyl group: These compounds and their common derivatives make up the bulk of organic compounds. Their common derivatives include acid halides:
acid anhydrides:
esters:
and amides:
Nomenclature of carboxylic acids
Two systems are used for naming carboxylic acids: the common system and the IUPAC system.
Common names for carboxylic acids are derived from Latin or Greek words that indicate one of their naturally occurring sources. Table 1 lists the common name, structure, source, and etymology for some common carboxylic acids. TABLE 1 Common Names of Carboxylic Acids Name Structure Source Etymology Formic acid
Ant
Formica (Latin) Acetic acid
Vinegar
Acetum (Latin) Butyric acid
Butter
Butyric (Latin) Caproic acid
Goat
Caper (Latin) Steric acid
Tallow
Steak (Greek) Employ the following steps to derive the IUPAC name for a carboxylic acid:
Pick out the longest, continuous chain of carbon atoms that contains the carboxyl group. The parent name for the compound comes from the alkane name for that number of carbon atoms.
Change the -e ending of the alkane name to -oic and add the word “acid.”
Locate and name any substituents, labeling their placement by numbering away from the carboxyl group.
Applying these rules gives the following compound the name 2-ethyl-4-methylpentanoic acid.
Naming carboxylic acid salts
Carboxylic acid salts are named in both the common and IUPAC systems by replacing the -ic ending of the acid name with -ate. For example, CH
(…)
… oxidation of primary alcohols leads to the formation of alde-hydes that undergo further oxidation to yield acids. All strong oxidizing agents (potassium permanganate, potassium dichromate, and chromium trioxide) can easily oxidize the aldehydes that are formed. Remember: Mild oxidizing agents such as manganese dioxide (MnO2) and Tollen's reagent [Ag(NH3)2+OH−] are only strong enough to oxidize alcohols…
… loses a proton to the nitrogen atom, forming an enol.
The enol tautomerizes to the more stable keto form.
The amide is protonated by the acid, forming a carbocation.
A water molecule is attracted to the carbocation.
The oxonium ion loses a proton.
The amine group is protonated.
An electron pair on one of the oxygens displaces the ammonium group from the molecule.
The carbonation of Grignard reagents…
… the substituted acid rather than the ketone.
A reaction between a disubstituted acetoacetic ester and dilute sodium hydroxide forms the following products:
Upon heating, the β ketoacid becomes unstable and decarboxylates, leading to the formation of the methyl ketone.
A Claisen condensation of ethyl acetate prepares acetoacetic ester.
The Claisen condensation reaction occurs by a nucleophilic…
… in an equilibrium condition and does not go to completion unless a product is removed as fast as it forms. The Fischer esterification proceeds via a carbocation mechanism. In this mechanism, an alcohol is added to a carboxylic acid by the following steps:
The carboxyl carbon of the carboxylic acid is protonated.
An alcohol molecule adds to the carbocation produced in Step 1.
A proton is lost from…
… out using a strong reducing agent, such as lithium aluminum hydride (LiAlH4). You can also use diborane (B2H6) to reduce carboxylic acids to alcohols. Reduction of esters
Esters are normally reduced by reaction with lithium aluminum hydride.
Reduction of acid halides
Acid halides are reduced by lithium aluminum hydride to primary alcohols.
Reduction of amides
Like other carboxylic acid derivatives…
... zobacz całą notatkę






Komentarze użytkowników (0)